Waa maxaydhul dhif ah?
Bini'aadamku waxay leeyihiin taariikh in ka badan 200 sano tan iyo markii la helay dhul dhif ah 1794. Tan iyo markii ay jireen wax yar oo macdanta dhulka ah oo la helay wakhtigaas, kaliya qadar yar oo ah oksaydhyada biyaha aan milmi karin ayaa lagu heli karaa habka kiimikada.Taariikh ahaan, oksaydhyada noocan oo kale ah ayaa caadiyan loogu yeeraa "dhulka", sidaas darteed magaca dhulka naadir ah.
Dhab ahaantii, macdanta dhulku naadir maaha dabeecadda.Dhul naadir ah maaha dhul, laakiin waa curiye bir ah oo caadi ah.Nooca firfircoonidiisu waa labaad ee biraha alkali iyo biraha dhulka alkaline.Waxay leeyihiin waxyaabo badan oo qolof ah marka loo eego naxaasta caadiga ah, zinc, tiin, cobalt, iyo nikkel.
Waqtigan xaadirka ah, dhulalka dhifka ah ayaa si weyn looga isticmaalaa qaybo kala duwan sida elektiroonigga, kiimikooyinka petrochemicals, metallurgy, iwm. Ku dhawaad 3-5 sano kasta, saynisyahannadu waxay awoodaan inay ogaadaan isticmaalka cusub ee dhulalka dhifka ah, lixdii ikhtiraac ee kasta, qofna ma samayn karo aan dhul naadir ah lahayn.
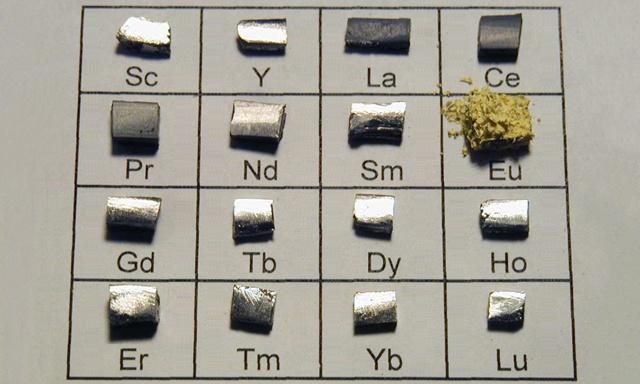
Shiinuhu waxa uu qani ku yahay macdanta dhulka naadirka ah,waxana uu kaalinta kowaad kaga jiraa sadexda qiimayn ee aduunka: kaydka, cabirka wax soo saarka,iyo mugga dhoofinta.Isla mar ahaantaana, Shiinuhu sidoo kale waa dalka kaliya ee bixin kara dhammaan 17 biraha dhulka naadir ah, gaar ahaan kuwa dhexdhexaadka ah iyo kuwa culus ee dhifka ah ee leh codsiyo militari oo aad u caan ah.
Halabuurka curiyaha dhulka naadir
Curiyayaasha dhulka naadirka ah waxay ka kooban yihiin curiyayaasha Lanthanide ee shaxda xilliyeed ee canaasiirta kiimikada:lanthanum(La),cerium(C),praseodymium(Pr),neodymium(Nd), promethium (Pm),samaroon(Sm),europium(Eu),gadolinium(Gd),terbium(Tb),dysprosium(Dy),holmium(Ho),erbium(Er),thulium(Tm),ytterbium(Yb),lutium(Lu), iyo laba walxood oo si dhow ula xidhiidha lanthanide:scandium(Sc) iyoyttrium(Y).

Waxaa la yiraahdaaDhulka naadir, oo loo soo gaabiyo Naadirka Dhulka.
Kala soocida curiyayaasha dhulka naadir
Lagu kala saaray sifooyinka jidheed iyo kiimikaad ee curiyeyaasha:
Curiyayaasha dhulka naadirka ah:Scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium
Cunsurrada dhulka dhifka ah ee culus:terbium, terbium, thulium, ytterbium, thulium, terbium, dysprosium.
Lagu kala saaray sifooyinka macdanta:
Kooxda Cerium:Lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium
Kooxda Yttrium:terbium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutium, scandium, yttrium
Kala soocida kala saarida:
Dhulka naadirka ah (P204 soo saarista aysidhka daciifka ah): lanthanum, cerium, praseodymium, neodymium
Dhul naadir ah oo dhexdhexaad ah (P204 soo saarid aysiidh yar):samarium, europium, gadolinium, terbium, dysprosium
Dhulka dhifka ah ee culus (soosaarka aysiidhka ee P204):Holmium, erbium, thulium, ytterbium, lutium, yttrium
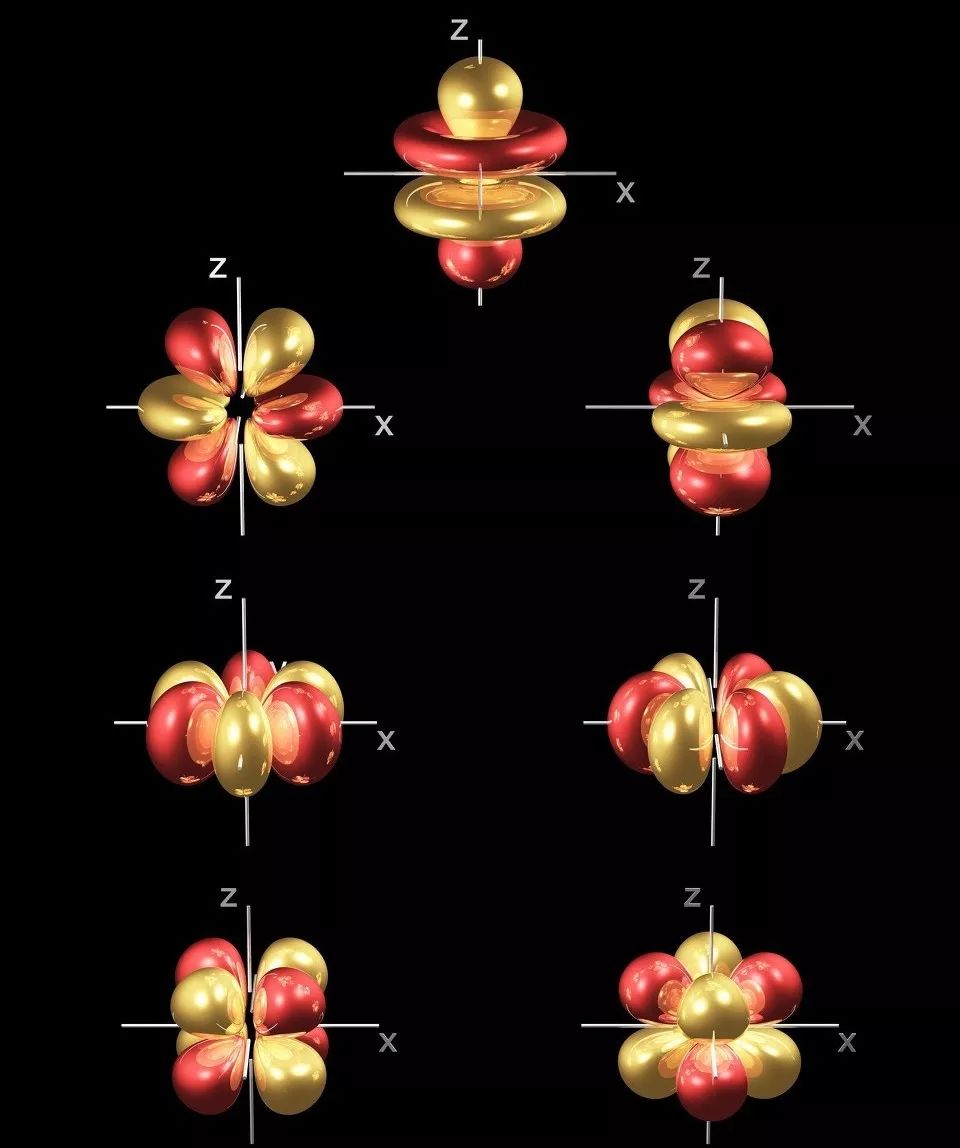
Astaamaha curiyayaasha dhulka naadir
In ka badan 50 hawlood oo curiyeyaasha dhulka naadir ah waxay la xiriiraan qaab-dhismeedkooda elektaroonigga ah ee 4f ee gaarka ah, taasoo ka dhigaysa in si ballaaran loogu isticmaalo agabka dhaqameed iyo agabka cusub ee tignoolajiyada sare.
1. Qalabka jirka iyo kiimikaad
★ Wuxuu leeyahay sifooyin macdan oo cad;Waa cawl qalin ah, marka laga reebo praseodymium iyo neodymium, waxay u muuqataa huruud khafiif ah
★ Midabo oksaydh qani ah
★ Samee xayndaabyo deggan oo aan biraha ahayn
★ Metal firfircoon
★ Si fudud hawada loo ogsixiyo
2 Alaabta indhaha
4f sublayer aan la buuxin, halkaas oo 4f elektaroonada ay ku gaashaaman yihiin electrons dibadda ah, taasoo keentay erayo kala duwan iyo heerarka tamarta
Marka 4f electrons u gudubto, waxay nuugi karaan ama soo saari karaan shucaaca hirarka hirarka kala duwan ee ultraviolet, oo u muuqda gobollada infrared, taasoo ka dhigaysa inay ku habboon yihiin qalabka iftiinka.
Habdhaqan wanaagsan, oo awood u leh diyaarinta biraha dhifka ah ee dhulka iyadoo loo marayo habka korantada
Doorka 4f Electrons ee Walxaha Dunida Naadirka ah ee Walxaha Cusub
1.Waxyaabaha isticmaalaya 4f sifooyinka elektarooniga ah
★ 4f habaynta wareegyada elektarooniga ah:Muujiyay sida magnetism xoog leh - ku habboon isticmaalka qalabka magnetka joogtada ah, qalabka sawirada MRI, dareemayaasha magnetic, superconductors, iwm.
★ 4f wareega elektarooniga orbitalWaxay u muuqataa sida guryaha luminescent - ku habboon in loo isticmaalo sida qalabka luminescent sida fosfooraska, lasers infrared, amplifiers fiber, iwm.
Kala-guurka elektiroonigga ah ee kooxda hagaha heerka tamarta 4f: waxay u muuqataa inay yihiin guryaha midabaynta - ku habboon midabaynta iyo midabaynta qaybaha meelaha kulul, midabada, saliidaha dhoobada, dhalooyinka, iwm.
2 waxa ay si dadban ugu xidhan tahay 4f elektaroonik, iyada oo la isticmaalayo raadiyaha Ionic, lacag iyo sifooyin kiimikaad
★ Astaamaha Nukliyeerka:
Qaybta nuugista nuugista kuleylka yar ee nuugista - ku habboon in loo isticmaalo sida qalabka dhismaha ee reactors nukliyeerka, iwm.
Qaybta nuugista neutron-ka weyn ee iskutallaabta - ku habboon ilaalinta agabka reactors nukliyeerka, iwm
★ Rare Earth Rare Ionic Radius, charge, physical and chemical properties:
Cilladaha Lattice, raadiyaha Ionic ee la midka ah, guryaha kiimikaad, kharashyo kala duwan - ku habboon kuleyliyaha, kicinta, walxaha dareenka, iwm.
Gaar ahaan qaab-dhismeed - ku habboon in loo isticmaalo sida agabka kaydinta hydrogen-ka ee walxaha cathode, agabka nuugista microwave-ka, iwm
Qalabka Electro indhaha iyo dielectric - ku habboon in loo isticmaalo sida qalabka wax-ka-beddelka iftiinka, dhoobada hufan, iwm.
Waqtiga boostada: Jul-06-2023